Ionic Magnesium – Food Grade
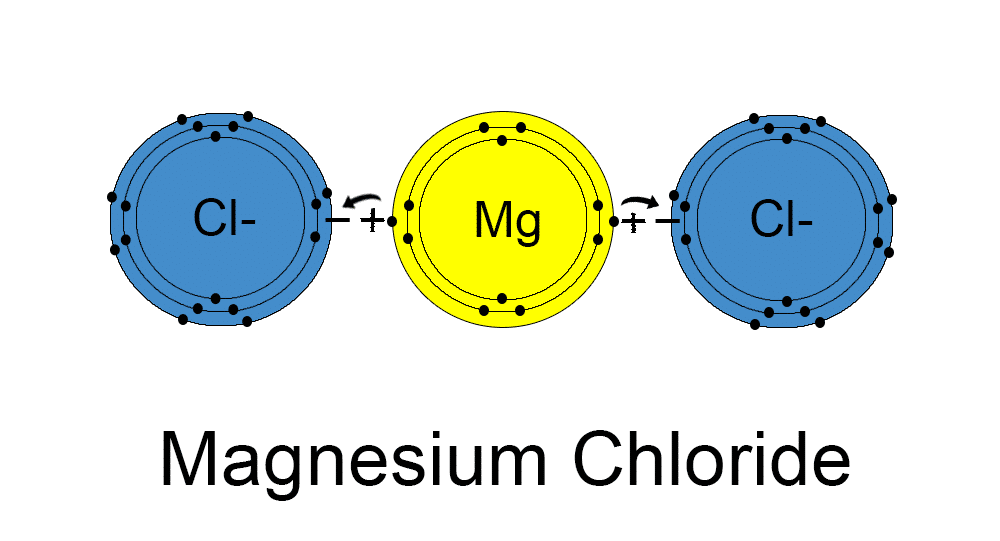
A similar ionic bonding happens with the other electrolyte salts to form potassium chloride, sodium chloride and calcium chloride. The most abundant mineral electrolyte in the body’s cells is the chloride ion – making up about 70% of the body’s total negative ion content . This means the body is very hungry for chloride and takes up magnesium chloride readily without further digestion. The compounding of chlorine with the essential light metals to form soluble ionic magnesium compounds (chloride compounds) is what makes them usable by cells, as they are in the salt form. Otherwise the metals are just colloidal; ie. particles suspended in solution, but not ionised with chlorine to become chloride.
Hydration and Electrolyte Charge
When a cell encounters mineralised ‘electrolyte’ water it soaks up more water because of the negative (alkaline) charge. This increased hydration effect enables more functional cell transport, ie. detoxification and movement of wastes out, as well as movement of nutrients into the cell. You need the minerals present to hold water inside the cell. Without enough magnesium charge the cell membrane becomes weak and leaky . Athletes in particular can lose large amounts of magnesium through strenuous exercise and perspiration. Without enough electrolytes to charge the system it becomes harder to maintain healthy hydration levels.
When we can hydrate better we also produce a stronger cell voltage to lift energy levels. Drinking filtered water with added magnesium chloride hexahydrate (food grade) minerals, including 2% trace minerals such as potassium, calcium, iron, boron, silica, strontium, chromium and minimal sodium, improves hydration (and supports bone health too!). About one pinch of flakes (1/2 a gram) per litre of filtered water makes a tasty mineral drinking water and lifts the pH of filtered water from 6 to 7 or over. If you want to lift alkalinity even higher (ie. 7 to 8) then add a tiny amount of sodium bicarbonate (bicarb soda). This well rounded electrolyte mix tastes great and makes the water easier to drink without gagging – or running to the toilet so often!
Cell Voltage and Energy
Electrolyte BALANCE is the key to good health, and magnesium is the master controller electrolyte regulating calcium channel firing and retreat. Calcium contracts and magnesium relaxes. Magnesium is also integral in the mitochondrial production of adenosine triphosphate (ATP) – the energy currency of the cell. We need magnesium to relax, to sleep, to detox, to digest, to exercise, to bolster the immune system, for hormone balance, for DNA repair and to build new cells. We need it for every process in the body that requires bio-electricity! Electrolyte salts dissolved in water enable electrical conductivity and cell voltage. Cell voltage is directly related to pH. Good electrolyte balance in cells and blood plasma results in an ideal pH status of 7.35-7.45 pH (slightly alkaline). Slightly alkaline means we have just the right level of surplus electrons to keep things going energetically.
Therefore, the body is hungry for chloride ions and magnesium chloride is very readily taken up by cells without requiring any further digestion. Once magnesium chloride is dissolved in water it is already in the right form for cellular uptake and effectively absorbed by cells everywhere – including the epidermal layer (as in transdermal absorption). No further digestion required.
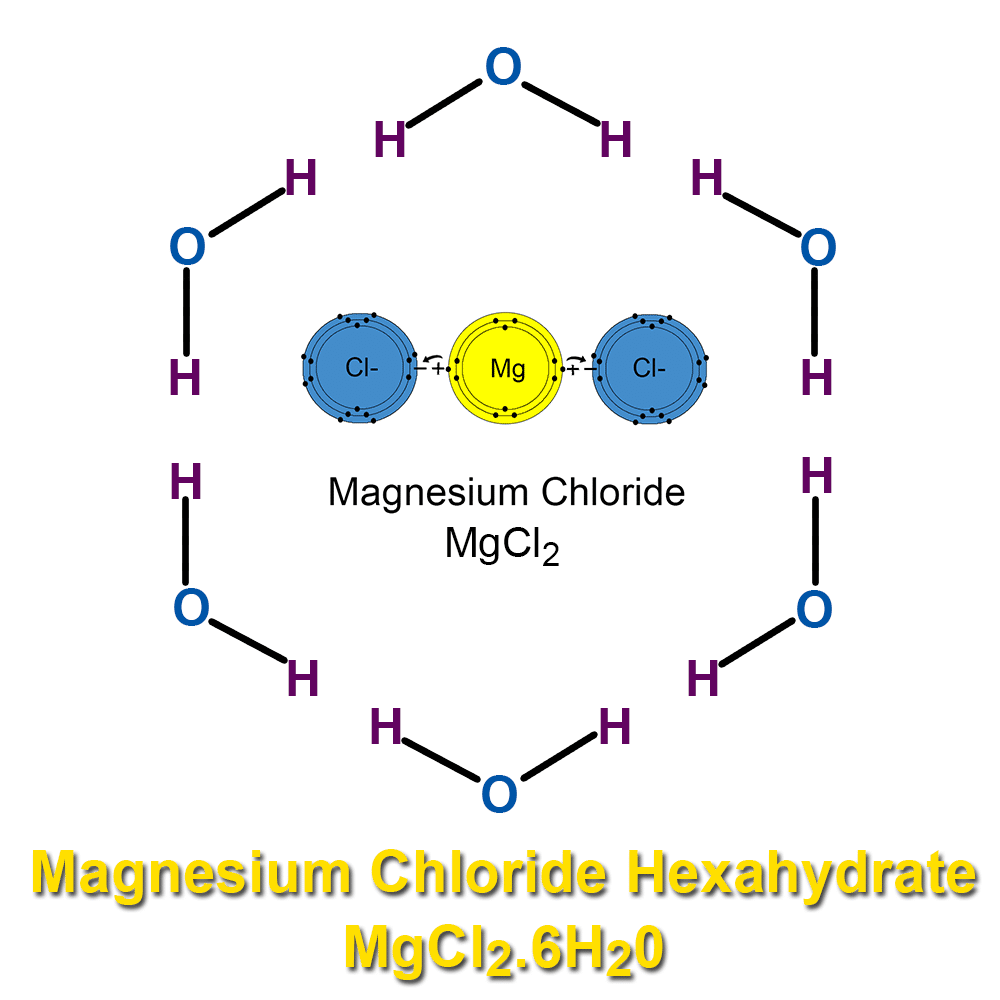

Even in its dehydrated crystalised flakes form magnesium chloride hexahydrate still is about half made up of water – ie. water molecules forming a crystalline structure. The other half of the atomic weight is approx 46-47% magnesium chloride plus 2-3% percent of mixed sea trace minerals. It is hygroscopic, hydrophilic, and water seeking: The salt will absorb moisture from the atmosphere until it eventually liquefies itself. It will always want to return back to its original liquid water state as soon as moisture is close by. Electrolyte charge attracts water molecules and life must have adequate hydration to flourish.
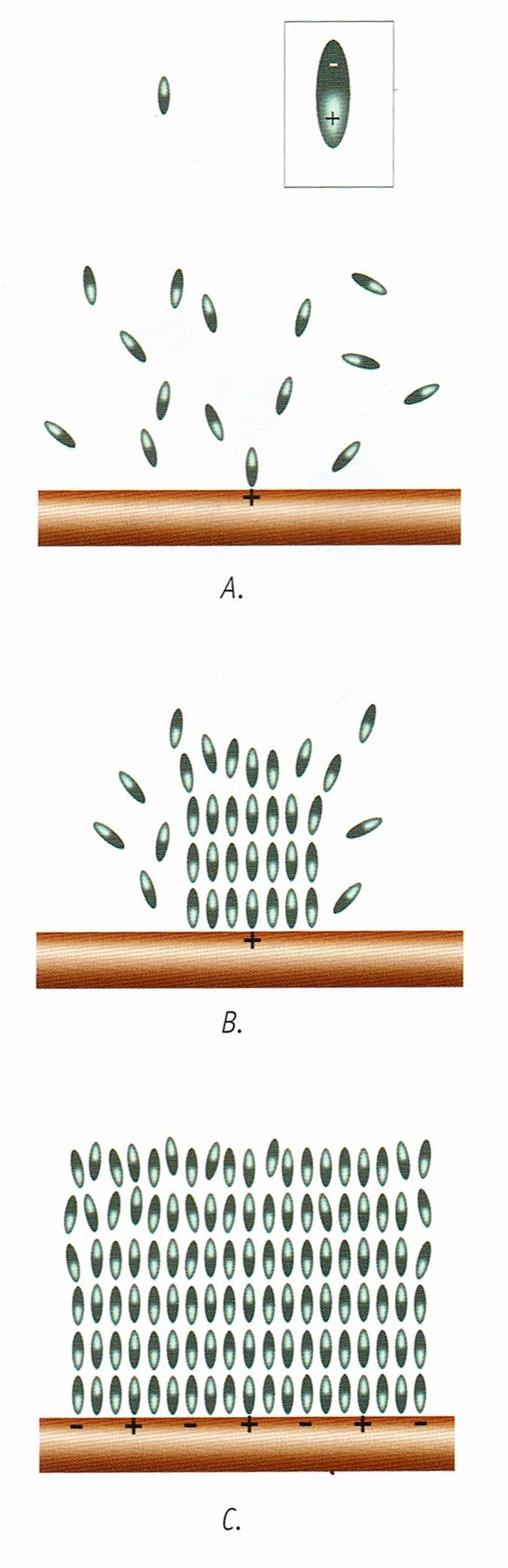
Structured Water
When water is charged with magnesium chloride electrolytes it orders the water molecules to assume a more structured form. That is, the salts cause the north-south poles of the water molecules to line up like train tracks which promotes alkalinity. This becomes part of the structure of the cell plasma and is why it feels slippery or gel-like.
This kind of charged water takes a bit longer to pass through the body as it is washing through the cells more effectively. Conversely, if you drink de-mineralised water (rain water, reverse osmosis or distilled) it goes though your digestive system faster, sending you frequently to the toilet. Your body will take this ’empty’ low pH water and look to steal electrons from somewhere else by scavenging for existing mineral electrolytes in your body to join up with in order to form ‘electrolyte water’ for cellular use and supply of electrons. This low pH demineralised water in a sense robs your body of minerals, and is one of the reasons why the medical profession is fearful of too much water. It’s not really the water we should be afraid of, but it’s not having enough minerals to go with it that requires caution – particularly magnesium which is commonly in short supply after stress.
Blood Fluidity and Cell Charge
It is essential to the health of our blood and circulation to have sufficient water and electrolyte balance. These days we tend to consume too much sodium and calcium, and not enough of the other electrolytes – particularly magnesium. Too much sodium and/or calcium can push your blood pressure too high, whereas magnesium has a normalising effect on blood pressure. Magnesium and potassium work in tandem as powerful intracellular electrolytes, whereas calcium and sodium ions are mostly extra-cellular due to the antagonism and competition of charges.
“Mg-deficiency is followed by a K-deficiency, which cannot be equalized by K alone: a refractory hypokalemia always needs additional Mg supply for its restitution. From K, Mg-deficiency a Na/Ca-overload of the cell with aggravating consequences will follow: impaired activity and vitality with electric instability. Mg, which is responsible for development of a Ca-overload is also able to restore electrolyte homeostasis by sufficient supply competitively.”
Magnesium is therefore integral to maintain cell wall integrity, electron charge/electrolyte balance, storage of ATP, and calcium channel regulation. If you lose too much magnesium the cell wall becomes weaker and you are more prone to cell dehydration and electrolyte depletion. This leads to platelet stickyness and positive charge (attractive force) – which is low in pH and therefore doesn’t supply enough electron flow. The negative charge of slightly alkaline however produces a repulsion of red blood cells so they bounce off one another and the blood becomes more fluid, thereby carrying more dissolved oxygen. More nutrients can also gain access to blood cells that are free and not clumped together because they have more surface area to absorb nutrients.
Relaxation of Muscle Fibres and Flexibility
Magnesium has been found to be the most effective natural calcium channel regulator. After calcium firing to contract muscle fibres, magnesium is used to relax the grip of the calcium. This relationship works a bit like a ‘zipper’ of muscle fibres closing and opening. When you are low in magnesium it becomes harder to open the zipper again and relax. This is expressed as cramps and locked up muscles and tendons.
What About Magnesium Tablets and Digestion?
Formulated tablets and powders containing compounds (ionic magnesium or chelated magnesium) must be digested in the stomach using hydrochloric acid. Many people today have issues with making enough stomach acid for digestion – especially when magnesium chloride levels are low (magnesium chloride is used to make stomach acid, as well as zinc, vitamin K, selenium and B group vitamins). Once the elemental magnesium is separated from the magnesium compound during digestion it needs to be recombined to form the magnesium chloride electrolyte for entry to the cell. Why not just use more magnesium chloride salts in the first place? Magnesium chloride in solution is already in the right form for cellular uptake.
In addition, these oral magnesium supplements often contain fillers and tablet coatings to keep them dry and stuck together, to transit and survive the stomach acid, or assist the coating to be more slippery for swallowing:
These magnesium tablets and powders can contain:
- Binders
- Disintegrants
- Fillers (diluents)
- Lubricants
- Glidants (flow enhancers)
- Compression aids
- Colors
- Sweeteners
- Preservatives
- Suspensing/dispersing agents
- Film formers/coatings
- Flavors
- Printing inks
Such additives can interfere with digestion and absorption of magnesium itself into the cell. Some people also suffer from clogged bowels, leaky gut syndrome or Crohns disease, which further compromise gut absorption of magnesium. Clinicians are finding that the average adult is lucky if they can even digest and absorb 20% of the magnesium from their swallowed tablets. At the lower end is magnesium oxide (commonly used in tablets) at only 4% bioavailability, with the better quality magnesium chelated compounds usually not absorbed more than 40% via the gut wall in the cleanest of gut digestive systems.
Chelated Magnesium
Chelated magnesium tablets are made in a laboratory combining elemental magnesium with an amino acid. ‘Chelating’ means joining together or grabbing onto. Chelated magnesium compounds are generally better tolerated by the digestive system than other forms of magnesium tablets without explosive diarrhea (as can happen with magnesium sulphate or magnesium oxide taken orally), but there is little evidence showing that it’s actually better digested or absorbed inside tissue cells. If your naturopath determines that you are low in other nutritional elements such as taurine, glycine, malic acid or other elements that are commonly compounded in the magnesium tablet, then by all means add those elements to your nutritional program. However, in the case of low magnesium status the body often needs a lot more magnesium than those tablets can provide.
Absorption and Bioavailability
There is only a certain concentration of magnesium that the gut wall can cope with absorbing at any one time, so it’s best to take in only the concentrations of magnesium provided by Nature in magnesium-rich fresh foods, microbiome-supporting fermented foods and mineral water containing magnesium. Note that our gut microbiome’s health relies heavily on magnesium availability (in natural forms), to digest foods, maintain the endothelial lining, for absorption of nutrients, maintenance of the immune system and production of neurotransmitters for brain function. Low magnesium has also been linked to depression.
In the case of oral supplementation, more is not necessarily better. If you take in higher concentrations of magnesium supplement it’s just excreted faster and you lose most in the toilet rather than having it penetrate the gut wall and gain access to tissue cells where most magnesium is used.
“Several authors have outlined that higher bioavailability is observed when a given amount of Mg is distributed over a day rather than being consumed in a single bolus (Schuette et al, 1990; Fine et al , 1991; Lonnerdal, 1995); consequently a regular water intake distributed throughout the day would be expected to lead to a higher absorption.”
“The low fractional absorption we observed for magnesium oxide (4 percent) is identical to that observed in prior studies of magnesium sulphate bioavailability… Results indicated significantly higher… bioavailability of magnesium chloride.”
Transdermal Magnesium
You can also gain magnesium by swimming in ocean water or soaking in mineral-rich hot springs. Transdermal Ionic Magnesium can bypass the digestive system altogether. The epidermal layer is able to absorb much higher concentrations of magnesium ions using dissolved magnesium chloride, as this form requires no further digestion. It’s faster acting and more efficient, and is often the only way to get large amounts of magnesium into the body in the case of leaky gut syndrome and other digestive disorders .
How Much Magnesium Mineral (Ionic Magnesium) Water Should We Drink?
This will vary a lot depending on your age, body size/shape, level of physical activity and perspiration, whether you are on medications, exposed to chemicals or under extreme stresses. Remember, water and minerals are the basis of the cell plasma, which determines our energy production using nutrients going in and waste products coming out. For a long time it was commonly thought that we are comprised of 70-75% water, however that is only measuring the volumetric liquids in our vessels and organs and fails to measure all of the water contained in the plasma of our cells, including the hard tissue of bone cells. Gerald Pollack in his water research has worked out we are 99% water molecules when all the moisture is added up:
“We are two-thirds water — by volume. In terms of the percentage of molecules, that two-thirds figure computes to a lot of water molecules:
more than 99% of our molecules are water molecules. Evidence suggests that those 99% don’t merely sit as the background carriers of the more important molecules of life, but are central participants. All that the cell does depends on water.” Professor Gerald Pollack
All life depends on water as the bio-electrical energy of life flows through it by virtue of its electrolyte content. If you are not supplying sufficient clean water with minerals, chances are your cells will dehydrate and become polluted and acidic, and consequently vulnerable to pathogenic attack and inflammation.
Inflammation and the Immune System
Inflammation is a result of the immune system going into overdrive to deal with the crisis of pathogenic attack (pathogens preferring low pH cellular conditions), and in the process produces swelling and phlegm in various places. When the body is dehydrated it also starts to get squeezed via the cardiovascular system and muscles to conserve water loss. Cramping, headaches and pain can result. The tubes in the body can become very clogged and sticky without enough electrolyte (magnesium) water.
Remember when you were young and your grandma made you chicken soup to treat a cold? It worked wonders – and has done for millennia. That’s because the bone and vegetable broth is rich in minerals (and essential fats) to help restore the gut microbiome and hydration in the body… You need a lot of mineralised liquids and liquid foods to recover from illness and to maintain wellness. Researchers are finding that an average of 2.5 to 3 litres of mineral water per 24 hours for an average adult is recommended for optimal hydration (and more if you perspire a lot).

Recommended Amount of Ionic Magnesium:
The recommended amount of elemental magnesium per adult per day is on average 400mg. Some people can need two or three times that amount to counteract the effects of trauma or chronic stress conditions. If you need higher amounts you are better off to absorb extra magnesium via skin (transdermally), as too much magnesium orally can cause loose stool or diarrhea.
Assuming you get only negligible amounts of magnesium from foods (common), to add even an average dose of magnesium to your diet you would need to consume 400mg of elemental magnesium in the form of magnesium chloride salts in your drinking water per day. This would be 2.5 grams of Elektra Magnesium flakes spread out in the drinking water supply over 24 hours, as there are approximately 160mg of elemental magnesium per gram of flakes. A dilution of 1/2 gram (one small pinch of 4-5 flakes) of magnesium chloride flakes per litre is easy on the digestive system as it is just a normal mineral drinking water amount. Three of these magnesium water litre bottles per 24 hours would therefore provide 1.5g of flakes (240mg of elemental magnesium ions). To arrive at 400mg orally you would need a further 160mg (one gram of Mg flakes). You can take in extra via magnesium mouthwash (mouthful after teeth brushing for healthy teeth and gums), or use magnesium mineral water to make cups of herbal tea or for cooking. Remember that magnesium chloride hexahydrate is a naturally occurring food ‘salt’ (not a drug or synthesized product). It doesn’t taste salty like sodium salt anymore because the sodium has been skimmed off during dehydration. Magnesium chloride salt tastes bitter on the tongue directly as flakes. However when you use it very diluted as mineral water it has a nice mineral water alkaline texture and taste.
Adding natural magnesium chloride to your drinking water is just adding natural magnesium as food salt to your diet, the same as you would add sodium chloride to your diet when you sprinkle sodium salt on your food for taste, or when you add herbs and spices to your cooking. It’s just food. Like with any food, you can also consume too much (concentrated) oral magnesium at one time, in which case you will notice a loosening of stool or diarrhea. In this case you are losing the magnesium too quickly from the digestive system before it has a chance to gain access to tissue cells. Your tissue cells may still be starving for more magnesium, but the digestive system is not able to cope with high-end delivery. Transdermal absorption allows better uptake for those with high-end needs.
Comparison With Other Liquid ‘Ionic Magnesium’ Solutions Using Magnesium Chloride:
I have seen quite a number of magnesium chloride supplement suppliers popping up in recent years around the planet as people start to realise how much we all need magnesium in our diets. They promote ‘Ionic Magnesium’ as though it were some kind of miraculous special potion they have invented and they charge the earth for it. At the end of the day it’s just magnesium salts and most commonly magnesium chloride hexahydrate flakes mixed with water – a natural salt compound developed by Nature. It is abundant on the planet – particularly in our oceans:

You can make magnesium electrolyte water and magnesium oil in your own home with the flakes at a fraction of the cost of what you pay for in a bottle. Just make sure that whoever you buy your ionic magnesium chloride (or ionic magnesium oil solution) from, that they can provide a laboratory analysis with testing down to 10ppb showing no mercury, no lead and no arsenic.
PRICE DIFFERENCE – BUYER BEWARE! One supplier I came across offered a 60mL bottle of ‘Ionic Magnesium’ which had a solution concentration of 100mg of elemental magnesium per one mL of liquid. The bottle was priced at US$10, which seems cheap on the face of it. At 400g per day of magnesium you would consume this bottle in 15 days. So, that’s US$10 every 15 days (plus shipping cost).
In comparison, Elektra Magnesium flakes would give you the same amount of Ionic Magnesium electrolyte liquid using 37.5 grams of flakes mixed with water to make 60mL. A 250g bag of Elektra Magnesium Flakes would fill 6.6 x 60mL bottles, giving you 6.6 x 15 days = 99 days worth at 400mg dose per day for only AUD$12.10 per 250g packet. If you buy a one kilo bag or a 4kg bucket the savings are even better. Go and do the maths.
By Sandy Sanderson © 2016 All Rights Reserved.
NOTICE: This article is for educational purposes only.
References:
