Tax included and shipping calculated at checkout
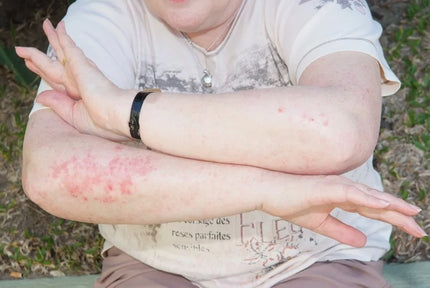
It’s easy these days to get caught on a treadmill of frenetic activity that you can’t get off until you hit the burnout button. Then you crash into a heap of fatigue that seems impossible to recover from. Your body feels heavy, you want to sleep but can’t, and you lose your mojo and enthusiasm to get anything done.

What is happening here is a crisis resulting from stress, trauma, sleep deprivation, toxic exposures, or excessive workloads (and maybe all of the above), which has severely depleted magnesium reserves. As magnesium is an essential component of the electrical nervous system, it’s like running out of spark plugs so there is nothing to ignite the engine to burn the fuel and perform tasks. It’s like unplugging your battery power. If you don’t have enough electrical ‘juice’ running through your system, it will slow down accordingly.
The body’s electrical system relies on sufficient water and magnesium to overcome fatigue and recover mitochondrial energy production. If you don't make enough electrical energy all systems run low, the body can't detox sufficiently, acidosis grows, fatigue becomes chronic, and eventually pain syndromes such as fibromyalgia can develop. Some people move further down the spiral into diabetes, neuropathy and cardiovascular dysfunction. Magnesium deficiency can even trigger cancer.
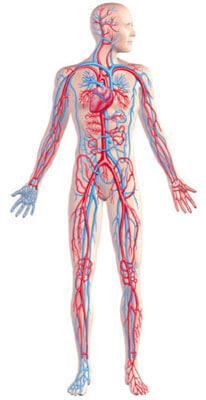
The main electrolytes involved in our cells’ electrical charge and conductivity are sodium, calcium, potassium and magnesium. These are ‘cations’, which means they have a positive charge (+) looking to join up with the opposite (-), that is, an anion which has a negative charge (ie. a spare space to accommodate the donated or shared proton+). As free dissolved ions they find each other during dehydration of salt water, connect and become a stable neutral crystalised salt molecule by sharing the surplus proton/s in the outer shell of the newly formed compound. The most common and abundant anion electrolyte in the body that joins up with a cation electrolyte is 'chloride' (=chlorine before it combines with the cation), thus forming; sodium chloride, calcium chloride, potassium chloride and magnesium chloride – all being electrolyte salts that are essential for our electrolyte charge, balance and metabolism. More about 'ionic magnesium' is in this article. Once the salts are dissolved again they become ionised and free from the crystalline salt compound.
The most prolific electrolytes inside cells are potassium and magnesium, whilst sodium and calcium exist mainly in the extra-cellular spaces. Potassium and magnesium are essential to help regulate cell transport systems, hydration and fluidity, as well as heart rhythm. Potassium deficiency symptoms are not likely until magnesium is first in short supply. This is because magnesium guards the cell gateways and helps the membrane to contain the potassium.
Excessive stress can cause increasing loss of magnesium via the kidneys. If there is not enough magnesium available to facilitate recovery, the charge of the cell membrane can continue to depolarise, causing potassium loss, as well as allowing excess sodium and calcium ions to enter cells and over-stimulate or dehydrate them.
The ebb and flow of electrolyte charge
Adrenalin surges during stress change the cell membrane charge potential, loosening the channels so that magnesium ions drop out and calcium ions move in for contraction of muscle fibres, or for neuronal firing in the brain. The recovery phase involves magnesium and hydration moving back in and displacing the calcium so that muscle fibres can expand and relax again. Because magnesium can dampen down adrenalin and control calcium, it is extremely important to the central nervous system, cardiovascular system, and muscles to get adequate magnesium.

The body can store extra magnesium in cell reservoirs for later use, and relies on reserves to compensate during times of stress or poor diet. Magnesium is most often stored in cells as the compound Mg-ATP, so that it is part of the biological battery of Adenosine Triphosphate, produced by mitochondria in metabolism.
99% of magnesium in the body is stored in the muscle and bone tissues. Only 1% is in the blood as a combination of free magnesium ions and stored magnesium in blood cells. Tissue cells can sacrifice stored magnesium to ensure the blood levels are in the normal range, as blood levels are crucial for the cardiovascular system. This is why blood tests are not an accurate indicator of whole-body magnesium status.
Calcium overload is called hypercalcemia and causes excessive ‘squeezing’ and cramps, muscle spasms, and tremors. It is a common sign of magnesium deficiency because magnesium controls how the calcium is used in the body, such as for the strengthening of bones and teeth. Without enough magnesium too much calcium can leech out of bones and become free calcium, causing havoc. Free calcium can settle in the arterial walls, or kidney tubules, which then lose flexibility.
One of the main reasons tissue cells can attract calcium deposits is due to some kind of inflammation and resulting drop in pH. The body tries to neutralise the acids via alkalising minerals. If not enough sodum bicarbonate and magnesium are available, calcium can be used for this purpose, as it also has a 2+ charge of two spare protons available to occupy the empty spaces of the free radical molecules. This bond then stabilises and neutralises the free radicals, bringing the pH of the cell environment back to normal.
If you use excessive vitamin D3, it can also attract more free calcium ions to settle in the soft tissues of the body, unopposed by magnesium. In this case we also need more magnesium as a counterbalance.
Magnesium is essential for mitochondrial production of our electrical energy currency ATP (adenosine triphosphate). Magnesium deficiency is the hallmark of metabolic syndrome, excessive and chronic fatigue, burnouts and energy crashes, diabetes and cardiovascular disease. Low magnesium means that metabolism suffers, resulting in electrical energy deficit, brain fog and lack of concentration, circulation issues and not enough oxygen delivery to cells. Sugar sensitivity increases as a direct consequence of magnesium deficiency, triggering states of acidosis and hypoxia.
This relationship is like a see-saw: As magnesium drops in cells, sugar sensitivity and cravings go up; and as you lift cellular magnesium stores, the sugar sensitivity and cravings diminish. You need 28 magnesium molecules to turn one surcose molecule into energy, so sugars make you waste a lot of magnesium. If you don't get enough magnesium to metabolise and burn the fat, your body has no choice but to switch to anaerobic (without oxygen) sugar metabolism to get any energy at all. But this kind of metabolism is more inefficient, and also produces more acidic byproducts, moving the body into a downward spiral trying to chase energy, but the acidity making it all harder to produce.
Natual magnesium salt and hydration
As the food supply is becoming more magnesium-depleted due to industrial farming methods and over-processing, and we also lose excessive magnesium under stress (and too many carbs), most people need to supplement with extra magnesium in order to help cope with the stresses, and recover from excessive fatigue.
It’s easy to get overwhelmed with all the magnesium supplement choices available, but if you want to go down the natural food pathway, then you can’t go past magnesium chloride (salt) flakes to supplement naturally. You can add it to drinking water and you can also soak it up via skin.
Caution is advised with diuretic drug therapies which can cause the kidneys to excrete too much water and too many alkalising minerals such as magnesium and potassium.

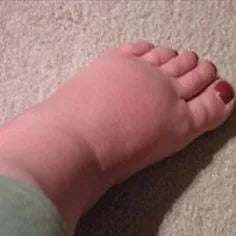
Water retention (oedema) in ankles can be a sign of dehydration and electrolyte deficiencies. As tissue cells become overloaded with waste toxins causing acidosis, and there is not enough water available for flushing, the brain signals to the kidneys to hold back sodium, which holds back water in the interstitial tissues, pooling generally in the ankles.
To support the body in this case, it is recommended by practitioners to cleanse, alkalise and rehydrate, and to do more exercise to move the blood circulation, as well as to excrete wastes via skin perspiration. Optimal hydration requires electrolytes in the drinking water, or the water is lost too quickly without adequately accessing the tissue cells. You can make a more hydrating and alkaline drinking water by adding magnesium chloride flakes and sodium bicarbonate to filtered water.
The most bioavailable form of magnesium

Natural magnesium chloride is the type of magnesium salt most easily dissolved in water and able to be directly taken up by cells without any further digestion required. You can soak in it as a bath or footsoak and get a good top up to your magnesium in just half an hour. Transdermal absorption works the fastest.
Magnesium ions are most commonly found inside our cells, as an intracellular cation. When you swallow other magnesium compounds they need to be first digested in the stomach with stomach acid so the body can extract magnesium and ionise it for cellular use. This takes a lot of work, resources and time (hours).

Opposites attract: Magnesium chloride, dissolved in water, turns magnesium swiftly into a free ion, ready for cells to use. Ionic magnesium has two spare protons in the outer shell, after the two chloride ions have dissociated (jumped off the protons that were being shared in the outer shell of the magnesium chloride salt compound). Magnesium chloride salt flakes therefore represent a docking system, offering the perfect natural delivery of magnesium to biological systems. More about ionic magnesium is in this article.
Note that the magnesium atom by itself is very stable and perfectly balanced with 12 electrons, 12 protons and 12 neutrons. Its atomic number is 12. But it cannot gain access to cells unless it is ionised, which means it has to lose two electrons in its outer shell, leaving two surplus protons (Mg2+) available to join with another element it encounters to make a new compound (like magnesium chloride). The cell needs both magnesuim ions and chloride ions, which can individually gain access to cells via the membrane channels. Because the body is so hungry for chloride, being the most abundant negatively charged ion in the body, and because magnesium chloride salt is so easily dissolved and made bio-available, it's an ideal nutritonal supplement.
Food grade is better than industrial grade magnesium chloride
Industrial grade magnesium chloride can be harvested from salt water of the Dead Sea, dessert salt pans, shallow lakes, or waste salts from desalination plants. It can have toxic metals or chemical residues from agricultural runoff, mining operations, fracking, PFOS (fluoride) fire-fighting chemicals, or sewage effluent leaks if near high density populations. If the magnesium chloride is mined out of a mountain rock cavity using solution mining techniques, the original source may be very pristine, but the process of mining can also introduce chemicals.
Industrial grade magnesium chloride is widely used to process magnesium metal alloys, for de-icing operations, to clean the air from dust in mining operations (because when the solution is sprayed in the air dust particles stick to it and drop to the ground), for aquaculture, aquariums and making bath salts.
Food grade magnesium chloride however is rarer, because, not only should the original salt water source be pristine, but the salt water needs to be harvested naturally from an open-air salt water source by siphoning and piping to dehydration tanks, so that no chemicals are introduced by drilling.
Food grade magnesium chloride (also called Nigari salt in Japan) is widely used by food manufacturers to make tofu, to remineralise filtered drinking water, and for use by pharmaceutical companies to further process into pharmaceutical grade which can be injected into the blood as a medicine.
The food grade magnesium chloride flakes that Elektra Magnesium uses are derived from open air salt water lakes 3,000m above sea level in the Tibetan mountain plateau. There are no added solvents or chemicals. They just pump the salt water down to the evaporation tanks. The more the salt water is evaporated, the more the sodium (NaCl) rises to the top of the brine and forms a crusty layer. The sodium chloride salt is then skimmed off and further refined to make table salt. The dehydration process continues until the remaining liquid brine, which is a thick salty slurry called ‘bischofite’, starts to form crystalline flakes.

This salt is called magnesium chloride hexahydrate. The chemical notation is MgCl2.6H20. It is comprised half of water molecules in a six-sided (hexahydrate) crystalline formation, with the balance being magnesium chloride 47-48%, plus about 2% residual trace minerals such as potassium, iron, calcium, boron, silica, strontium etc., a trace mineral package a-la-Nature to buffer and enhance our overall electrolyte energy needs.
Elektra Magnesium flakes are also further tested by an Australian laboratory to 10 parts per bilion to ensure there are no mercury nor lead contaminants, and that they comply with the certified food grade international standard.
Why does magnesium oil feel sticky and irritating?

Magnesium oil is formed when magnesium chloride flakes are dissolved in extra water (rehydrated) until a slippery viscose texture is achieved (usually between 30 and 70% concentration in the salt water solution). The skin’s lipid barrier is a natural water repellent, so magnesium chloride salt in strong solution can sit on the top of skin for too long, feeling sticky, irritating. and drying out the skin too much.
Soaking in a magnesium bath facilitates magnesium absorption because the hot water helps to open the skin channels, and the concentration of magnesium to water is relatively low. The body is always looking to balance water with salts.
When applying a strong magnesium chloride solution directly to skin, transdermal absorption is easier when natural lipids (fats) are also present, because the skin loves fats and absorbs them readily (together with the magnesium ions) until the skin reservoir is full. Therefore the condition and hydration of the skin greatly influences the absorbability of magnesium.

Elektra Magnesium skin and muscle care range stands out from the crowd because of the unique proprietary method of fusion of magnesium chloride salt with natural plant oils, butters and extracts without the use of petrochemical derivatives or synthetic emulsifiers and preservatives. Elektra Magnesium products offer comfortable skin-friendly ways to supplement magnesium and recover from stress and fatigue, catering for a variety of skin types, needs and age groups from babies to elderly.
As the epidermis reaches full saturation well before any risk of overdose, the body is always in control. You can use as much as you like. The skin simply takes up the magnesium it needs in its own time, and receives additional antiaging and skin conditioning benefits. Refer to Magnesium Dose Guide on FAQ page for more information.
MAGNESIUM IS THE MASTER MINERAL ELECTROLYTE, acting as a conductor of an orchestra of nutrients... If magnesium crashes, other systems follow because of the energy deficit.
REFERENCE RE DIURETICS: https://pubmed.ncbi.nlm.nih.gov/3732091/ “Hypokalaemia and hypomagnesaemia can be induced by the same mechanisms and are often clinically correlated with one another. The reported incidence of hypomagnesaemia is greater than that of hypokalaemia; a significant correlation also appears to exist between the plasma concentrations of magnesium and potassium. A significant inter-relationship between the plasma concentrations of magnesium and potassium and the evidence for a critical role of magnesium in the genesis of cardiac arrhythmias would support the proposal that magnesium should be routinely measured in situations, such as diuretic therapy, that are potentially associated with hypokalaemia.”